| -
|
|
 |
BIO GREEN
AMMONIA,
100% GREEN AND ORGANIC NITROGEN
This project, now a reality, is perhaps the most ambitious
we have developed. It is said that only 14% of the ammonia used as
fertilizer is consumed by humans through food. The rest ends up on
the ground or in the air.
Emissions in the absence of human
interference are 0.7 kg annually per hectare/year. Modern
agriculture has multiplied this figure by 25, causing the
modification of the natural nitrogen cycle with the consequences of
the contamination of soils, waters and the acidification of the
oceans.
Today we have the solution that replaces the Haber-Bosch
process, used successfully for more than 100 years, but which did
not consider environmental protection factors. |
|
|
|
BIOMIMETIC PRODUCTION OF NITROGEN FROM NATURAL ORIGIN
AND PLANTS.
Bio Green Ammonia is a reality, the project
is the most advanced technology that exists for the production of
nitrogen for agricultural use. Bio Green Ammonia, means
reducing to zero:
- All CO2 emissions generated
in the production of Nitrogen..
- All NH3 emissions generated in the production of
Nitrogen.
And more:
- Capture, store and reuse CO2 from the
atmosphere.
-
Produce nitrogen for agricultural use 100% ECO & ORGANIC. |
|
|
|
BIO GREEN AMMONIA MEANS PRODUCTION OF GREEN NITROGEN AND FERTILIZERS
WITH ZERO CARBON EMISSIONS |
|
 BACKGROUND BACKGROUND
The nitrogen
used in agriculture basically comes in two forms: ammonia and urea
and derivatives of both.
AMMONIA
Ammonia is produced naturally through the
decomposition of organic matter, but the truth is that currently,
and for more than 80 years, the wrong large-scale industrial model
was created. The process used to obtain ammonia is the Haber-Bosch
process and its name comes from the first chemists who carried it
out: Fritz Haber and Carl Bosch.
UREA
The synthesis reaction for urea production involves
combining ammonia with CO2 under pressure to form
ammonium carbamate, which decomposes into urea and water. Unreacted
CO2 is recirculated. The main raw material to obtain this
compound is natural gas, which through a chemical process is first
converted into ammonia and then dehydrated to form, for example,
urea.
|
|
But what if we dispense with the synthesis industry
and natural gas and produce ORGANIC NITROGEN, 100% NATURAL, in a
sustainable and non-polluting model…
For this, there is currently only one model
and one possibility, Bio Green Ammonia, GREEN NITROGEN.
This private project, developed by KERVRAN LABS, is
developed in Malaga, Andalusia, SPAIN with a proprietary
technology (patent pending) called the UJADOS-GIL PROCESS,
which replaces and surpasses the Haber-Bosch process for the
following reasons, among many others:
- Energy consumption is minimal and is carried out with a
solar electric motor also developed for the project, which
does not require large photovoltaic infrastructures, just a
small installation of 20 square meters.
- Fossil fuels are not used, nor any other type of fuel, LPG,
hydrogen.
And the project is a reality that has already passed the
laboratory/prototype and pre-industrial phases, and is in
the beginning of production phase.
BIO GREEN AMMONIA IS A REALITY.
|
 |
|
|
 TWO
CONCATENATED METHODOLOGIES FOR ONE PRODUCT TWO
CONCATENATED METHODOLOGIES FOR ONE PRODUCT
Biomimicry and non-polluting models..
§
PROCESS 1
EXTRACTION THROUGH BASIC SOLUBILIZATION.
In the organic matrix of plant tissues that are used
as raw materials (fabaceae and legumes) where the nutrient to be
obtained is found: NITROGEN associated with other macro and
micronutrients in a complex form. It also naturally incorporates
percentages of iron, calcium and silicon. This association is
possible thanks to the fact that all these elements are found in an
organic matrix that allows their natural compatibility.
In production,
they are only used as pH modifiers or complements to natural
processes; short-chain carboxylic acids of plant and/or food origin
are used.
Under no circumstances are synthetic chemical
solvents USED, nor are EDTA, EDHA, ligno-sulfonic acid or any other
similar chelators used.
With this model, values of up to 5% or slightly
higher can be reached, depending on the nitrogen contained in the
fresh plant tissue and other factors such as temperature, extraction
speed, pH of the water, etc.
§
PROCESS 2
EXTRACTION BY CAPTURE OF ATMOSPHERIC CO2.
Nitrogen is captured from the air using a MECHANICAL
SYSTEM of polymeric membranes that allow the rapid passage of one
gas while minimizing the passage of other gases when a pressure
gradient is applied across the membrane. In this way, the membrane
separates oxygen and other “fast gases” from compressed air and thus
generates a nitrogen stream of 95% purity..
PRINCIPIOS DE FUNCIONAMIENTO
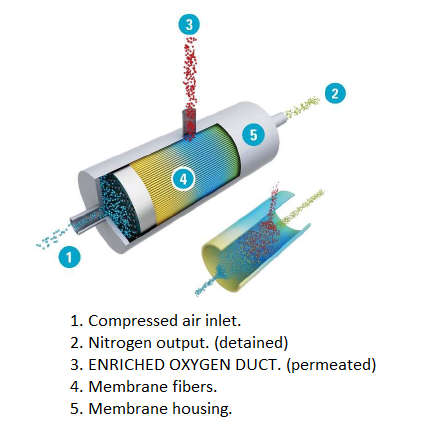 |
Air from the atmosphere is introduced through the air
filter, passes through the air inlet valve and enters the
compression element.
This compressed air is forced through the air
separator, past the minimum pressure valve and into the reservoir (on
TM units).
The purities of nitrogen obtained from air by mechanical separation
range from 95% to 99.5% (residual oxygen content from 5% to 0.1%).
The generator consists of one or several high-performance membranes.
This membrane configuration ensures maximum output of
compressed nitrogen for minimum input of compressed air, over a wide
range of operating conditions.
To allow continuous operation these nitrogen generators are equipped
with a top quality 2-stage pre-filtration system that guarantees an
inlet air quality according to ISO8573-1:2010.
This eliminates the risk of membrane damage from poor quality
compressed air supply, poor starts and unexpected stops. |
|
|
|
The plant tissue that was used in PHASE 1 and from
which the maximum possible nitrogen has been obtained, remains as an
amalgam of decomposing organic matter that is stored as a base to
create a natural ecosystem similar to that of a peat bog.
Peat forms as a result of the partial rotting and carbonization of
vegetation in the acidic water of swamps, marshes, and wetlands. The
formation of a peat bog is relatively slow as a consequence of low
microbial activity, due to the acidity of the water or the low
concentration of oxygen.
The passing of the years produces an accumulation of
peat that can reach several meters thick, at a growth rate that is
estimated to be between half and ten centimeters every hundred years.
But... what if we vary the conditions with a BIOMIMETICAL
model applying the following vectors?:
Promoting that water, instead of an acidic environment, is in an
alkaline environment. With plant species that do it naturally.l.
-
Enhancing the microbial
activity of water in a state of putrefaction, the “peatlands” in
production ponds used for production, with organisms that belong
to the subgroup of proteobacteria, which includes the following
genera: Allorhizobium, Azorhizobium, Bradyrhizobium,
Mesorhizobium, Rhizobium and Sinorhizobium, Ensifer and others
with similar biological behavior.
-
Conducting captured
atmospheric nitrogen to this particular ecosystem to enhance the
activity of microorganisms and their fixation in water.
It happens that we are in an environment similar to that of a peat
bog, but with a greater potential for nitrogen fixation in the water
and a much higher rate, and can even reach values of 50% of nitrogen
solubilized in water.
And what
happens to the C02 that is produced in the biological
production processes of Bio Green Ammonia?
It is also captured and
consumed by nitrogen-fixing microorganisms and by plant organisms
that develop on the surface of the “production peatlands.” Another
fraction is taken to pools for the production of calcium carbonate
in a biomimetic and natural way.
Biological
Nitrogen Fixation (BNF)
As is known, nitrogen
fixation by bacteria occurs when they convert gaseous nitrogen from
the air and CO2 into inorganic compounds. Although the
role of legumes is undeniable, the task is too difficult for them
alone. In fact, it is normal for symbiotic nitrogen fixation to
occur between legumes and bacteria. It is common for Rhizobium and
others to colonize the roots.
However, it is not the only
symbiotic nitrogen fixation in legumes: biological nitrogen fixation
also occurs with other associated and free organisms, which are
those found in the substrate of the peat bogs used in our
Bio Green Ammonia production
process. which is successively decanted to the bottom of the water
under the substrate until the desired percentage is reached..
|
|
|
 |
-
IT IS A SUSTAINABLE MODEL WHOSE ONLY
RESIDUAL EMISSION IS OXYGEN FROM THE SEPARATION OF
ATMOSPHERIC NITROGEN.
-
NO CHEMICAL SOLVENTS ARE USED.
-
IT'S AN ENERGY SELF-SUFFICIENT MODEL,
EQUIPPED WITH AN ENERGY PRODUCTION UNIT WITH AN
ELECTRICITY GENERATOR WHICH IS USED FOR PUMPING AND
OPERATION OF THE NITROGEN CAPTURER.
-
DOES NOT REQUIRE SOLAR PANELS, COMPLEX
INSTALLATIONS.
-
DOES NOT CONSUME FOSSIL FUELS.
-
IT DOES NOT PRODUCE WASTE, NEITHER SOLID
OR LIQUID.
-
CAPTURES, STORES AND REUSES
ATMOSPHERIC CO2, GENERATING OTHER SUSTAINABLE PRODUCT
AND INDUSTRIAL MODELS..
|
|
|
|
Agricultural Inputs (final products) that incorporate
CERTIFIED ORGANIC NITROGEN produced by Bio Green Ammonia. |
 |
 |
 |
|
 |
Join the Natural and 100% Sustainable Agriculture model
of ORGANIC NITROGEN produced by Bio Green Ammonia..
|
-
Farmer or
agricultural producer who wants to use the organic and
sustainable products of Bio Green Ammonia to
create a NATURAL AGRICULTURE model.
-
Fertilizer
Manufacturerthat want to use green and organic nitrogen
as an ingredient in your products or formulations..
-
Marketer or
Distributor that want to distribute
Green and Organic
Nitrogen..
-
Financial
investor who wants to participate in a key future
project for the environment and agriculture..
 Contact
now:
bga@biogreenammonia.com Contact
now:
bga@biogreenammonia.com |
| |
|
|
|
|
|
Copyright 2023 © KERVRAN LABS
Tecnología y Consultoría Medioambiental
28806 Málaga. Spain |
|
The Environmental Technologies Experts. |













